When to ask male adolescents to provide semen sample for fertility preservation?
Introduction
Current epidemiologic studies estimate that 12,060 new cancer cases were expected to occur among children 0 to 14 years of age in 2012, and childhood cancer incidence rates are increasing at a rate of 0.5% per year from 2004 to 2008 (1). According to Surveillance, Epidemiology and End Results (SEER) data, 9% of patients diagnosed with cancer from 2004 to 2008 were younger than 45 years old, with “childhood” malignancy estimated to affect up to 1/168 Americans aged 15-30 (2,3). While the incidence rates for some forms of childhood cancer have increased since the mid-1970s, death rates have declined dramatically for most childhood cancers and survival rates have markedly improved (4). Due to the improvements in the treatment regimens, cancer patients are exhibiting better outcomes, longevity and potentially cure or remission. With the average cancer patient expected to have prolonged survival, physicians should counsel patients on the long term adverse effects of cancer treatment, including the preservation of fertility. It is known that certain chemotherapy and radiotherapy treatment regimens can lead to infertility, and efforts to assess the success of fertility preservation in children who are at risk of fertility impairment or sterility due to cancer treatment are currently underway. A report from the Childhood Cancer Survivor Study showed that patients who had undergone cancer treatment and were not surgically sterile were half as likely to contribute to a pregnancy when compared to their siblings (5). These serious long-term adverse effects of oncologic therapy in adolescent and young adult males and females who would otherwise expect to lead a normal life after surviving cancer, including raising a family of their own, can have serious consequences on the remainder of their adulthood (6). The concern for future fertility of adolescents is not limited to cancer patients: Klinefelter syndrome (KS) patients who appear to have a decline in their fertility potential shortly after the onset of puberty (7), as well as patients who are being treated with cytotoxic medication for conditions such as Systemic lupus erythematosus (SLE) or benign renal diseases that can also impair germ cells and reproductive organs.
Currently, both the American Society for Reproductive Medicine (ASRM), and the American Society of Clinical Oncology (ASCO) recommend providers treating both adult and pediatric malignancy discuss the possible impact of cancer and cancer therapies on fertility, make early referrals of interested patients to reproductive specialists, and address fertility preserving options such as sperm cryopreservation when appropriate (8,9). Despite these recommendations, most oncologists do not consistently discuss fertility preservation options with patients at risk for developing treatment-related infertility in fear of delaying cancer treatment (10), and among the adolescents that are given the option of fertility preservation, the success rate of sample collection for sperm preservation is not optimal because the lack of counseling (11). Currently, most guidelines lack specific direction on when clinicians should initiate discussion with adolescents to self-stimulate and provide a semen sample for cryopreservation. Very few studies have evaluated the success rate of masturbation to provide semen sample for cryopreservation in adolescents and in the published literature there is a substantial variability in the sperm retrieval rates from attempts at masturbation for cryopreservation. Multiple reasons can explain this variability: i.e., Being embarrassed and uncomfortable about producing a semen sample, or being too ill to do so (12). Moreover, an important factor that can contribute to this variability is knowing the appropriate age when to ask adolescents to masturbate and the capacity to ejaculate and produce a semen sample. Historically, there have been very few studies that explore this topic. This limitation in published literature may stem in part from the belief that exploration of this intimate behavior is too difficult or too invasive given social norms and cultural barriers around sexual privacy. Kinsey et al. were among the first to document masturbatory practices in 1948. Their published work showed that most men and 62% of women masturbated at some point in their lifetimes (13). Since then, most studies have focused on how many people masturbate, how often, how they learned, and what effect masturbation had on their sexual behaviors and development. The objective of our study was to explore the age at which adolescents begin puberty, masturbating, ejaculating, and to evaluate the age limits of when to ask an adolescent to provide a semen sample for cryopreservation.
Materials and methods
We prospectively evaluated healthy volunteers at a single tertiary-care center in the United States according to an Institutional Review Board (IRB)-approved protocol. All subjects underwent an initial screening for orgasmic, erectile, ejaculatory function, general health, sexual history, and history of sexual development. Volunteers responded to the American Urological Association (AUA)/International Prostate Symptom Score (IPSS), International index of erectile function (IIEF)-15, and Male Sexual Health Questionnaire (MSHQ) questionnaires to rule out any confounding medical or urological history. Volunteers were excluded if the AUA/IPSS >1, IIEF-15 less than the maximum score in each of the areas, or less than 5 on any item in the MSHQ. Our inclusion criteria were men between the ages of 18 and 65 years with normal erectile, ejaculatory, orgasmic, and voiding function who do not use prescribed or over-the-counter medications. All subjects had capacity to provide informed consent and no psychiatric history. All subjects were compensated for participation in the study.
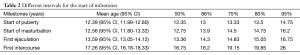
All 50 men were asked four questions to self-report the age of the onset of puberty, when they first started to masturbate, their first experienced ejaculation, and their first sexual intercourse (Table 1). Patients were provided definitions of each sexual activity and puberty, then given adequate time to report their deliberated response. Puberty was defined as hair growth under the arms, on the face and in the pubic area, as well as growth of the penis, testicles, and scrotum (with reddening and folding of the skin).

Full table
Statistical analysis, included descriptive analysis of the study group, lognormal exponential to determine the cumulative percentage when each milestone occurs, linear regression to test for correlation between the onsets age of each of the milestones. And t-test was used to compare the mean response between the two age groups using JMP® v 10.0.2.
Results
Fifty men between the age of 18-65 (mean age 39 years, ±16.03) volunteered for the study. The mean reported age for the start of puberty was 12.39 years (95% CI, 11.99-12.80 years). The mean age of the first experienced ejaculation was 13.59 years (95% CI, 13.05-14.12 years). When the cohort was asked about masturbation, the mean reported age for the start of masturbation was 12.56 years (95% CI, 11.80-13.32 years), and the first episode of intercourse was at the mean age of 17.26 years (95% CI, 16.18-18.33 years). Seventy five percent of the cohort reached puberty by the age of 13.33 years, and 95% of the men surveyed reached puberty by the age of 14.75. And when asked about the start of masturbation, 75% of the cohort reported that they started by the age of 14.5 and 95% started to masturbate by the age of 16.2. In this cohort, 75% experienced their first ejaculation by the age of 14.83, and 95% were able to ejaculate by the age of 16.75. Moreover, 75% experienced their first intercourse at the age of 19.15 and 95% of the cohort had that experience at the age of 26 (Figure 1,Table 2).
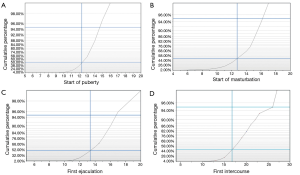
Full table
The first experienced ejaculation fell one year and six months after the onset of puberty in 80% of the cohort; and 84% started masturbation one year and six months after the onset of puberty. To account for any recall bias, the cohort were separated into younger and older age groups [18-30 (n=25) and 45-65 (n=25) years, respectively]. When we compared the means in their response to the questionnaires, there was no statistical significance between the two groups. Additionally, on age distribution analysis, the mean age for the start of puberty is reached before the majority of the cohort first experienced ejaculation, and first experienced intercourse (Figure 2). We further examined the correlation between the milestones reported age of onset for the four variables. The first experienced ejaculation was positively correlated with age of onset of masturbation [R2 =0.33 (P<0.0001)]. Furthermore, ejaculation was positively correlated with the first intercourse [R2 =0.17 (P=0.002)], and the onset of puberty [R2 =0.14 (P=0.01)] (Figure 3).
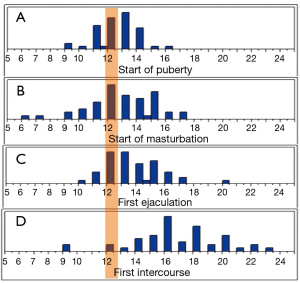
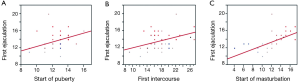
Discussion
It is recognized that certain chemotherapy, radiotherapy, and cytotoxic treatment regimens can lead to irreversible declines in sperm production, and pre-treatment attempts at sperm cryopreservation may be critical for future fertility (5). The decline in fertility potential for adolescents and young adult males who would otherwise expect to lead a normal life can have serious consequences on the reset of their adulthood. Male infertility can occur as a result of cytotoxic damage to the rapidly differentiating spermatogonia during cancer treatment from a wide variety of chemotherapeutic agents (14,15). The extent of testicular damage and recovery are both specific to the chemotherapeutic agents and the treatment dose used. Gonadal damage usually peaks at six months (16), it can persist up to two years and in patients receiving alkylating agents the impairment can be lifelong (17). Similarly, radiotherapy induced testicular damage and the time for sperm count to return to pretreatment levels are also both dose-dependent (18,19). During chemotherapy and/or radiation, the germ cells may be both quantitatively and qualitatively damaged (17). Additionally, the undesirable effects of those therapies depends on intrinsic characteristics of the patient, such age (20), the acuity of illness, the genetic predisposition of the germ cells to damage, and tumor biology (21,22). It is clear that the male germinal epithelium is very sensitive to chemotherapy and radiation; therefore, adolescents, and their parents have to be counseled that infertility is a likely side-effect with these therapies. For these patients, sperm cryopreservation should be offered, since the majority of male cancer survivors wants their biological offspring post-treatment (23,24).
Traditionally, semen collection procedures include abstinence for a minimum of 48 hours before ejaculation and the gathering of multiple samples (up to 3) to maximize the amount of sperm available for cryopreservation, especially if patients have suboptimal semen samples. Because of the large variability present in semen parameters (25), it is extremely difficult to correlate semen quality with any sexual or development milestones. Hence, there is a limited knowledge of semen parameters and sperm quality in young adolescents. Of all the factors that can affect successful semen sample collection, spermarche is the most important in determining the success of obtaining a sample that contains sperm. Spermarche tend to occurs around the age of 13, and can range from age 12 to the age of 15 (26,27). The reported age of spermarche correlated with the reported age, based on interviews, of first conscious ejaculation (28). Any adolescents around this age who have experienced ejaculation should be considered a candidate for self-stimulation for collecting a semen sample. It is important to individualize patient management, recognizing that disease status may adversely affect sperm numbers or quality, and that some adolescents who ejaculate will have azoospermia. These are components of counseling that should be provided before attempts at cryopreservation. However, advances in assisted reproductive technology have recently changed the semen quality parameters necessary for fertilization, obviating the need for a high pre-freeze sperm density (29). As a result, perfect semen parameters are not always necessary.
Limited studies have previously investigated the success rate of masturbation in adolescents to provide semen samples for cryopreservation, and the success rates are usually good. Postovsky et al. evaluated the feasibility of sperm cryopreservation in 27 male adolescents between the ages of 14 and 19 years. Eighty eight percent (24/27) were successful in self-stimulating and delivering a sample for sperm preservation (30), and all patients that ejaculated had sperm in the ejaculate. Another study evaluated the success of semen sampling in 86 adolescents (aged 12.2-17.9 years) showed similar success rates. Of the 86 boys, 74 (86%) produced a semen sample by masturbation, and 88.4% had spermatozoa in their ejaculate (31). Similar results were reported in other studies on semen sampling in adolescent cancer patients (32,33). Despite the reported success, only 19% of male cancer patient bank sperm before treatment (23). And almost half of oncologists either never mention sperm banking or offer it to less than a 25% of their patients who will receive cancer treatment potentially damaging to fertility (10). The lack of confidence by the practitioner obtaining useful samples from adolescents, and the difficulty due to the sensitivity of the topic to discuss with adolescent patient and their families have discouraged practitioners from embracing the practice of offering cryopreservation (10). Moreover, the disparities in the stages of maturity, since puberty may take place anywhere from the age of 10 to 14 years (34), and the lack of guidance about the age of when to approach patients for semen collection, may cause confusion among some practitioners and further discourage counseling.
In our study we demonstrated that the first experienced ejaculation occurred one year and six months after the onset of puberty in 80% of the cohort; and 84% will start masturbation one year and six months after the onset of puberty. We also found that the average adolescent will reach puberty before reaching other sexual milestones, specifically, first experienced ejaculation and intercourse. Therefore, the use of puberty plus 1.5 years will be a good tool to use in predicting the age of when successful ejaculation might occur. And since first conscious ejaculation is correlated to the reported age of spermarche (28), this might also predict the success rate of sperm collection. Our reported mean age for the onset of puberty, first experienced ejaculation, and start of masturbation are consistent to what is reported in the literature (35,36). However, in our study we demonstrated that the first experienced ejaculation and masturbation as well as puberty were positively correlated; as it is demonstrated in published studies that looked at sign of puberty such as testicular growth and the onset of puberty (37). One might argue that simply by making adolescents comfortable and obtaining a detailed history pertinent to past practices of masturbation and ability to ejaculate, is important in predicting the success of sample collection for semen analysis and cryopreservation.
Many of the subjects are asked to recall features of their development from as many as 20 years prior, and it is possible that the results may contain an element of recall bias. However, when we stratified the patients into older and younger groups there was no statistical different between the two groups. Studies that tested the validity of self-reported age of menarche found it to be comparable with that of recorded age (38), and the discrepancies in the reported timing of specific milestone events can be as low as two months (39). It is also possible that subjects with relatively late onset of milestones may be less likely to volunteer for this study, as this may contribute to an element of shame.
The issue of fertility preservation and semen cryopreservation is also relevant in the adolescent KS population. Early sperm retrieval from semen or testicular tissue has been suggested by several investigators (40). KS patients have optimal testicular function after the onset of puberty until mid-puberty, and decline thereafter (41). In some cases, sperm may be present in the ejaculate for young men at this age of 12-14 years, and it have been reported that sperm was found in 70% of ejaculated semen samples from adolescents with KS aged 12-20 years (7). However, sperm are routinely found in the testes of 70% of adult men with KS at our institution (42), so it is not critical that sperm be retrieved early unless treatment that can suppress sperm production such as testosterone therapy is required. Therefore, it is arguable at the onset of masturbation and ejaculation, fertility preservation for KS adolescents should be considered to cryopreserve sperm to potentially avoid future invasive surgeries in adulthood.
Conclusions
Our data showed that the onset of puberty proceeded the first experienced ejaculation by one year and six months in 80% of our cohort; and 84% will start masturbation one year and six months after the onset of puberty. The first experienced ejaculation was positively correlated with age of onset of masturbation. Thus it is appropriate to consider a request for semen specimens by masturbation from teenagers at one year and six months after the onset of puberty. Perhaps the onset age of puberty plus 1.5 years is an important predictor of ejaculation and sample collection for cryopreservation.
Acknowledgements
Funding: This study was funded by grant no: H6D-MC-X009 from Lilly USA, LLC and unrestricted research support from Irena and Howard Laks.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Society AC. Cancer Facts & Figures 2012. Atlanta, 2012.
- Bleyer A. Young adult oncology: the patients and their survival challenges. CA Cancer J Clin 2007;57:242-55. [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute, 1975-2008. seercancergov/csr.
- Ries LA, Smith MA, Gurney JG, et al. eds. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. SEER Program 1999; NIH Pub. No. 99-4649.
- Green DM, Kawashima T, Stovall M, et al. Fertility of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2010;28:332-9. [PubMed]
- Langeveld NE, Stam H, Grootenhuis MA, et al. Quality of life in young adult survivors of childhood cancer. Support Care Cancer 2002;10:579-600. [PubMed]
- Mehta A, Paduch DA. Klinefelter syndrome: an argument for early aggressive hormonal and fertility management. Fertil Steril 2012;98:274-83. [PubMed]
- Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in cancer patients. Fertil Steril 2005;83:1622-8. [PubMed]
- Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24:2917-31. [PubMed]
- Schover LR, Brey K, Lichtin A, et al. Oncologists’ attitudes and practices regarding banking sperm before cancer treatment. J Clin Oncol 2002;20:1890-7. [PubMed]
- Bashore L. Semen preservation in male adolescents and young adults with cancer: one institution’s experience. Clin J Oncol Nurs 2007;11:381-6. [PubMed]
- Greenberg ML, Urbach SL. Preserving the fertility of children with cancer. Med J Aust 2006;185:532-3. [PubMed]
- Kinsey AC, Pomeroy WR, Martin CE. Sexual behavior in the human male. 1948. Am J Public Health 2003;93:894-8. [PubMed]
- Costabile RA. The effects of cancer and cancer therapy on male reproductive function. J Urol 1993;149:1327-30. [PubMed]
- Cicognani A, Pasini A, Pession A, et al. Gonadal function and pubertal development after treatment of a childhood malignancy. J Pediatr Endocrinol Metab 2003;16 Suppl 2:321-6. [PubMed]
- Byrne J, Mulvihill JJ, Myers MH, et al. Effects of treatment on fertility in long-term survivors of childhood or adolescent cancer. N Engl J Med 1987;317:1315-21. [PubMed]
- Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr 2005.12-7. [PubMed]
- Centola GM, Keller JW, Henzler M, et al. Effect of low-dose testicular irradiation on sperm count and fertility in patients with testicular seminoma. J Androl 1994;15:608-13. [PubMed]
- Gordon W Jr, Siegmund K, Stanisic TH, et al. A study of reproductive function in patients with seminoma treated with radiotherapy and orchidectomy: (SWOG-8711). Southwest Oncology Group. Int J Radiat Oncol Biol Phys 1997;38:83-94. [PubMed]
- Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA 1988;259:2123-5. [PubMed]
- Meirow D, Schenker JG. Cancer and male infertility. Hum Reprod 1995;10:2017-22. [PubMed]
- Agarwal A, Said TM. Implications of systemic malignancies on human fertility. Reprod Biomed Online 2004;9:673-9. [PubMed]
- Schover LR, Rybicki LA, Martin BA, et al. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer 1999;86:697-709. [PubMed]
- Schover LR, Thomas AJ, Falcone T, et al. Attitudes about genetic risk of couples undergoing in-vitro fertilization. Hum Reprod 1998;13:862-6. [PubMed]
- Jarow JP, Fang X, Hammad TA. Variability of semen parameters with time in placebo treated men. J Urol 2013;189:1825-9. [PubMed]
- Guízar-Vázquez JJ, Rosales-López A, Ortiz-Jalomo R, et al. Age of onset of spermaturia (spermache) in 669 Mexican children and its relation to secondary sexual characteristics and height. Bol Med Hosp Infant Mex 1992;49:12-7. [PubMed]
- Ji CY. Age at spermarche and comparison of growth and performance of pre- and post-spermarcheal Chinese boys. Am J Hum Biol 2001;13:35-43. [PubMed]
- Schaefer F, Marr J, Seidel C, et al. Assessment of gonadal maturation by evaluation of spermaturia. Arch Dis Child 1990;65:1205-7. [PubMed]
- Kuczyński W, Dhont M, Grygoruk C, et al. The outcome of intracytoplasmic injection of fresh and cryopreserved ejaculated spermatozoa--a prospective randomized study. Hum Reprod 2001;16:2109-13. [PubMed]
- Postovsky S, Lightman A, Aminpour D, et al. Sperm cryopreservation in adolescents with newly diagnosed cancer. Med Pediatr Oncol 2003;40:355-9. [PubMed]
- Hagenäs I, Jørgensen N, Rechnitzer C, et al. Clinical and biochemical correlates of successful semen collection for cryopreservation from 12-18-year-old patients: a single-center study of 86 adolescents. Hum Reprod 2010;25:2031-8. [PubMed]
- Bahadur G, Ling KL, Hart R, et al. Semen quality and cryopreservation in adolescent cancer patients. Hum Reprod 2002;17:3157-61. [PubMed]
- Menon S, Rives N, Mousset-Siméon N, et al. Fertility preservation in adolescent males: experience over 22 years at Rouen University Hospital. Hum Reprod 2009;24:37-44. [PubMed]
- Nielsen CT, Skakkebaek NE, Richardson DW, et al. Onset of the release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair, and height. J Clin Endocrinol Metab 1986;62:532-5. [PubMed]
- Smith AM, Rosenthal DA, Reichler H. High schoolers masturbatory practices: their relationship to sexual intercourse and personal characteristics. Psychol Rep 1996;79:499-509. [PubMed]
- Auslander BA, Rosenthal SL, Blythe MJ. Sexual Development and behaviors of adolescents. Pediatr Ann 2005;34:785-93. [PubMed]
- Carlier JG, Steeno OP. Oigarche: the age at first ejaculation. Andrologia 1985;17:104-6. [PubMed]
- Cooper R, Blell M, Hardy R, et al. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health 2006;60:993-7. [PubMed]
- Fisher CM. Assessing Developmental Trajectories of Sexual Minority Youth: Discrepant Findings from a Life History Calendar and a Self-Administered Survey. J LGBT Youth 2012;9:114-35. [PubMed]
- Forti G, Corona G, Vignozzi L, et al. Klinefelter’s syndrome: a clinical and therapeutical update. Sex Dev 2010;4:249-58. [PubMed]
- Wikström AM, Raivio T, Hadziselimovic F, et al. Klinefelter syndrome in adolescence: onset of puberty is associated with accelerated germ cell depletion. J Clin Endocrinol Metab 2004;89:2263-70. [PubMed]
- Ramasamy R, Ricci JA, Palermo GD, et al. Successful fertility treatment for Klinefelter’s syndrome. J Urol 2009;182:1108-13. [PubMed]


